ExoMAX™ Opti Enhancer
- High purity—supports separation of exosomes from viruses and protein aggregates
- High yield—delivers more exosomes than the traditional protocol, start with smaller samples
- More hands-free—three simple steps before the density gradient
- Flexible—compatible with downstream biomarker discovery and functional assays
- Scalable—pellet exosomes from any volume of conditioned media or biofluid and resuspend as needed for the density gradient
Products
| Catalog Number | Description | Size | Price | Quantity | Add to Cart | |||
|---|---|---|---|---|---|---|---|---|
| EXOMAX12A-1 | ExoMAX™ Opti Enhancer | 12 Reactions | $429 |
|
||||
| EXOMAX24A-1 | ExoMAX™ Opti Enhancer | 1 Kit | $629 |
|
||||
Overview
Overview
Easier preparation for density gradient ultracentrifugation
For researchers needing highly pure exosomes, sucrose or OptiPrep™ (iodixanol) density gradient ultracentrifugation are the methods of choice. However, sample preparation prior to the density gradient is a time-consuming and multi-step process. To streamline the pre-density gradient steps, SBI has developed ExoMAX™ Opti Enhancer, an easy-to-use reagent that can move samples to the density gradient in three easy steps.
- High purity—supports separation of exosomes from viruses and protein aggregates
- High yield—delivers more exosomes than the traditional protocol, start with smaller samples
- More hands-free—three simple steps before the density gradient
- Flexible—compatible with downstream biomarker discovery and functional assays
- Scalable—pellet exosomes from any volume of conditioned media or biofluid and resuspend as needed for the density gradient
"We have tested the ExoMAX reagent. It gave us 5-fold more yield than conventional ultracentrifugation in a side-by-side comparison." —Zongdi Feng, Nationwide Children's Hospital
References
How It Works
How It Works
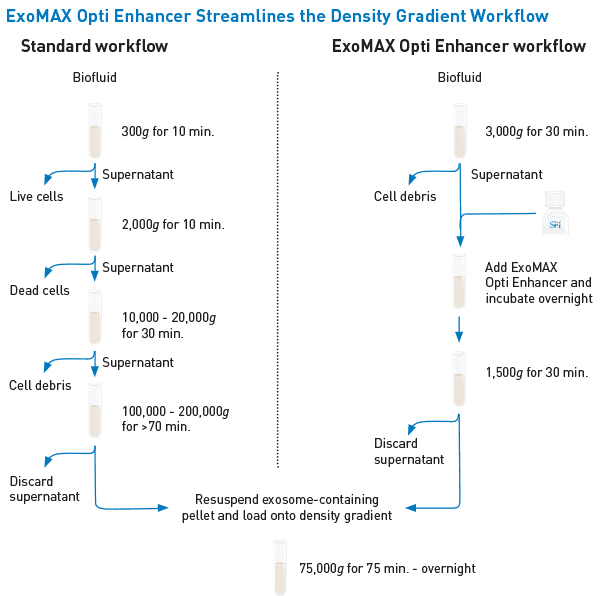
Get to the gradient fast
Instead of multiple low speed centrifugation steps followed by a high-speed ultracentrifugation step (left panel), with ExoMAX Opti Enhancer you can simply centrifuge the cell culture medium or biofluid to pellet cellular debris, incubate with ExoMAX Opti Enhancer, centrifuge again, and load the resuspended pellet onto the density gradient—no preliminary high-speed spin necessary (right panel). The resulting exosomes harvested from the density gradient are present in higher amounts compared to standard preparation methods, allowing you to start with smaller sample volumes, and can be easily separated from other co-precipitating particles such as viruses and protein.
The streamlined ExoMAX Opti Enhancer workflow—a better way to get to the gradient.
Supporting Data
Supporting Data
High yield exosome isolation from virus-infected cells
To demonstrate the excellent performance of ExoMAX Opti Enhancer, we isolated exosomes from HIV-infected T-cells. Five mL of conditioned medium (for ExoMAX protocol) and 10 mL of conditioned medium (for standard UC protocol) from infected cells were collected, and vesicles isolated using either ExoMAX Opti Enhancer reagent (Figures 1 and 2, top panels) or the standard protocol (Figures 1 and 2, bottom panels) before separation using OptiPrep gradient medium and ultracentrifugation at 110,000g for 70 minutes.
Fractions from the gradient were collected and lysed for subsequent Western blot analysis, which show exosome yield (Figure 1), and separation of exosomes from virus particles (Figure 2). Compared to the standard density ultracentrifugation workflow, ExoMAX Opti Enhancer delivers a higher yield of exosomes (Figure 3).
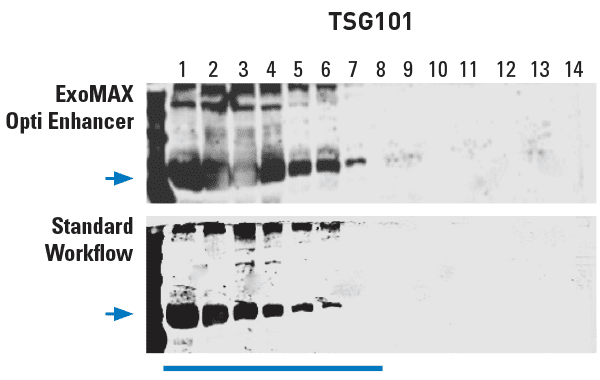
Figure 1. ExoMAX Opti Enhancer delivers high yields of exosomes. Density gradient fractions probed with exosome-specific anti-Tsg101 antibody show exosomes in fractions 1-7, with higher exosome yields from the ExoMAX Opti Enhancer workflow from a 5 mL media sample (top panel) than in the standard workflow from a 10 mL media sample (bottom panel).
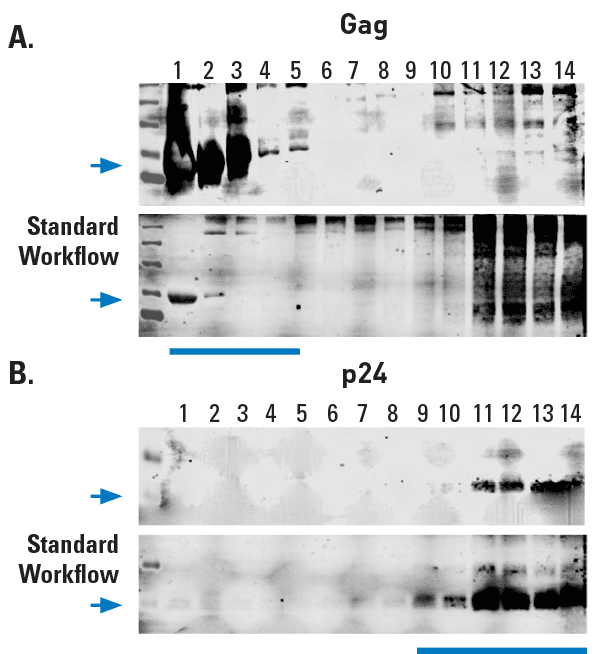
Figure 2. Exosomes prepared using ExoMAX Opti Enhancer can be purified away from virus. The HIV Gag protein is known to be abundant in exosomes from infected cells1 whereas the p24 capsid protein is only found in assembled virus. (A) Density gradient fractions probed with anti-Gag antibody show the presence of Gag in the same fractions (fractions 1-5) that contain exosomes (exosome-containing fractions are identified in Figure 1). (B) However, HIV virus, as indicated by the presence of the HIV p24 capsid protein, is only detected in the non-exosome-containing fractions 11-13.
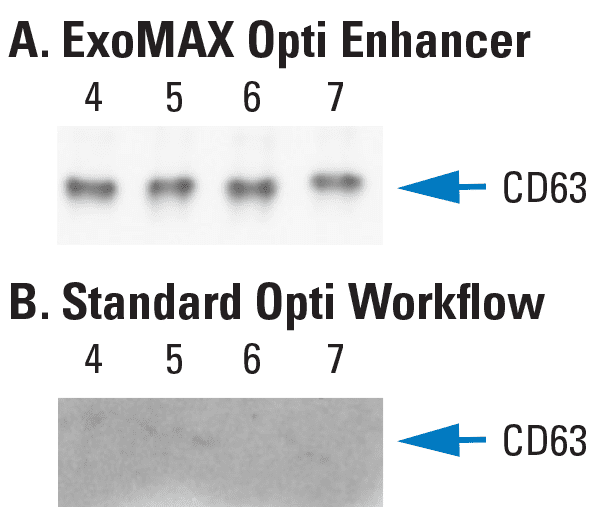
Figure 3. Higher yields of exosomes are obtained from OptiPrep-based ultracentrifugation when samples are prepared using ExoMAX Opti Enhancer compared to the conventional sample preparation workflow. Prior to OptiPrep-based ultracentrifugation, the same volume of sample (10 mL) was prepared using either (A) ExoMAX Opti Enhancer or (B) the standard workflow. Western blots of selected ultracentrifugation fractions probed with antibodies to CD63, an exosomal marker, show that while 10 mL of sample is sufficient to obtain exosomes when using ExoMAX Opti Enhancer, it is not sufficient volume to purify exosomes using the standard OptiPrep workflow. Data provided courtesy of Dr. Fatah Kashanchi, George Mason University.
References
- Narayanan A, et al. Exosomes derived from HIV-1-infected cells contain trans-activation response element RNA. J Biol Chem. 2013; 288(27):20014-33. PMCID: PMC3707700.
FAQs
Documentation
Citations
Related Products
Products
| Catalog Number | Description | Size | Price | Quantity | Add to Cart | |||
|---|---|---|---|---|---|---|---|---|
| EXOMAX12A-1 | ExoMAX™ Opti Enhancer | 12 Reactions | $429 |
|
||||
| EXOMAX24A-1 | ExoMAX™ Opti Enhancer | 1 Kit | $629 |
|
||||
Overview
Overview
Easier preparation for density gradient ultracentrifugation
For researchers needing highly pure exosomes, sucrose or OptiPrep™ (iodixanol) density gradient ultracentrifugation are the methods of choice. However, sample preparation prior to the density gradient is a time-consuming and multi-step process. To streamline the pre-density gradient steps, SBI has developed ExoMAX™ Opti Enhancer, an easy-to-use reagent that can move samples to the density gradient in three easy steps.
- High purity—supports separation of exosomes from viruses and protein aggregates
- High yield—delivers more exosomes than the traditional protocol, start with smaller samples
- More hands-free—three simple steps before the density gradient
- Flexible—compatible with downstream biomarker discovery and functional assays
- Scalable—pellet exosomes from any volume of conditioned media or biofluid and resuspend as needed for the density gradient
"We have tested the ExoMAX reagent. It gave us 5-fold more yield than conventional ultracentrifugation in a side-by-side comparison." —Zongdi Feng, Nationwide Children's Hospital
References
How It Works
How It Works
Get to the gradient fast
Instead of multiple low speed centrifugation steps followed by a high-speed ultracentrifugation step (left panel), with ExoMAX Opti Enhancer you can simply centrifuge the cell culture medium or biofluid to pellet cellular debris, incubate with ExoMAX Opti Enhancer, centrifuge again, and load the resuspended pellet onto the density gradient—no preliminary high-speed spin necessary (right panel). The resulting exosomes harvested from the density gradient are present in higher amounts compared to standard preparation methods, allowing you to start with smaller sample volumes, and can be easily separated from other co-precipitating particles such as viruses and protein.
The streamlined ExoMAX Opti Enhancer workflow—a better way to get to the gradient.
Supporting Data
Supporting Data
High yield exosome isolation from virus-infected cells
To demonstrate the excellent performance of ExoMAX Opti Enhancer, we isolated exosomes from HIV-infected T-cells. Five mL of conditioned medium (for ExoMAX protocol) and 10 mL of conditioned medium (for standard UC protocol) from infected cells were collected, and vesicles isolated using either ExoMAX Opti Enhancer reagent (Figures 1 and 2, top panels) or the standard protocol (Figures 1 and 2, bottom panels) before separation using OptiPrep gradient medium and ultracentrifugation at 110,000g for 70 minutes.
Fractions from the gradient were collected and lysed for subsequent Western blot analysis, which show exosome yield (Figure 1), and separation of exosomes from virus particles (Figure 2). Compared to the standard density ultracentrifugation workflow, ExoMAX Opti Enhancer delivers a higher yield of exosomes (Figure 3).
Figure 1. ExoMAX Opti Enhancer delivers high yields of exosomes. Density gradient fractions probed with exosome-specific anti-Tsg101 antibody show exosomes in fractions 1-7, with higher exosome yields from the ExoMAX Opti Enhancer workflow from a 5 mL media sample (top panel) than in the standard workflow from a 10 mL media sample (bottom panel).
Figure 2. Exosomes prepared using ExoMAX Opti Enhancer can be purified away from virus. The HIV Gag protein is known to be abundant in exosomes from infected cells1 whereas the p24 capsid protein is only found in assembled virus. (A) Density gradient fractions probed with anti-Gag antibody show the presence of Gag in the same fractions (fractions 1-5) that contain exosomes (exosome-containing fractions are identified in Figure 1). (B) However, HIV virus, as indicated by the presence of the HIV p24 capsid protein, is only detected in the non-exosome-containing fractions 11-13.
Figure 3. Higher yields of exosomes are obtained from OptiPrep-based ultracentrifugation when samples are prepared using ExoMAX Opti Enhancer compared to the conventional sample preparation workflow. Prior to OptiPrep-based ultracentrifugation, the same volume of sample (10 mL) was prepared using either (A) ExoMAX Opti Enhancer or (B) the standard workflow. Western blots of selected ultracentrifugation fractions probed with antibodies to CD63, an exosomal marker, show that while 10 mL of sample is sufficient to obtain exosomes when using ExoMAX Opti Enhancer, it is not sufficient volume to purify exosomes using the standard OptiPrep workflow. Data provided courtesy of Dr. Fatah Kashanchi, George Mason University.
References
- Narayanan A, et al. Exosomes derived from HIV-1-infected cells contain trans-activation response element RNA. J Biol Chem. 2013; 288(27):20014-33. PMCID: PMC3707700.