
Best practices
Perhaps all of this interesting research has got you excited about extracellular vesicle isolation and the potentials thereof.
There are a few things you may want to consider though.
The first of which revolves around the confusion about, and lack of consensus of, how to distinguish different subsets of EVs from one another.
Luckily in 2014, the International Society for Extracellular Vesicles (ISEV) came up with the minimal experimental requirements for the definition of extracellular vesicles.
In summary, the guidelines state that—if you’re going to characterize single vesicles—you should use two different yet complementary techniques when doing so. For instance: electron or atomic force microscopy to have an image of the EVs in order to assess their size and shape; and single particle tracking (NTA) where one can assess both the size and count.
ISEV’s requirements for general characterization include:
- ≥ 3 positive protein markers for EVs, with at least one being a:
- Transmembrane/lipid-bound protein
- Cytosolic protein
- ≥ 1 negative protein marker such as argonaute or histones that shouldn’t be contained within exosomes.
Given that, let’s presume you’d like to focus your extracellular vesicle isolation efforts specifically on exosomes. Maybe you’re on the hunt for exosomal biomarkers or you’re looking to actually use known exosome-based biomarkers for a downstream purpose.
What are some of the other important things you should keep in mind when harnessing these exosomes as biomarkers?
Well, for starters let’s consider a basic exosome research workflow (Diagram 3.1).
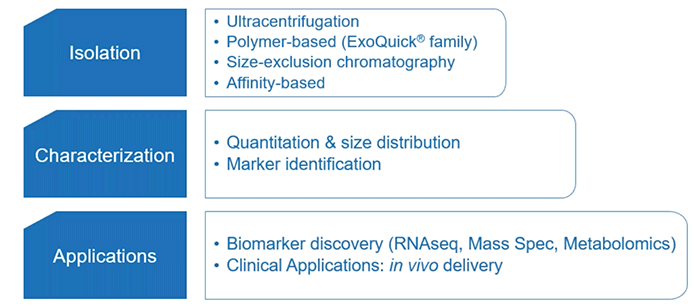
Diagram 3.1: Exosome Research Workflow.
From SBI
You can break this workflow down into three main sections: extracellular vesicle isolation, characterization, and application; including biomarker discovery and clinical applications.
To keep things brief, we’ll highlight some of the important considerations of the first part: extracellular vesicle isolation.
With respect to extracellular vesicle isolation, there are various methods one can rely upon. But irrespective of your method of choice—such as ultracentrifugation or size exclusion chromatography—the pre-treatment of your sample (serum or plasma) is what’s important.
The main difference between serum and plasma is that plasma is normally considered to be a more physiological fluid. That being said, it requires some wise thinking on your part when it comes to choosing an anticoagulant. Normally, chelating agents are recommended as opposed to heparin, which may lead to interference in PCR.
And with either serum or plasma, you must consider your choice of pre-clearing (e.g. centrifugation).
So what do you think is most often the deciding factor for choosing serum vs. plasma then? Well, the answer might surprise you.
Most often, the choice of serum vs. plasma is based on what’s available! Even when you have a choice between the two, it’s wise to think about any potential limitations of using one vs. the other within your experimental design.
Download the Ebook
Click here to download the pdf version of the ebook


