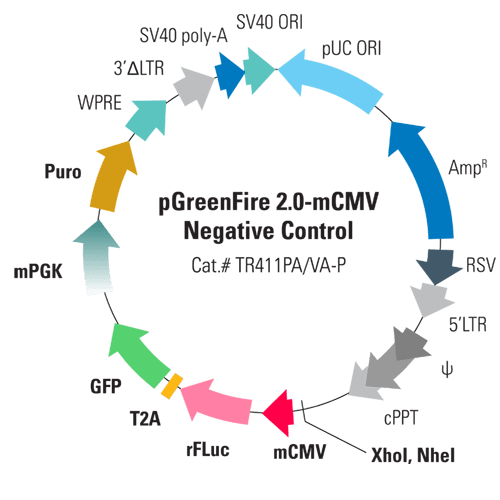
pGreenFire 2.0 mCMV Cloning & Negative Control Lentivector & Virus
Products
| Catalog Number | Description | Size | Price | Quantity | Add to Cart | |||
|---|---|---|---|---|---|---|---|---|
| TR411PA-P | pGreenFire 2.0-mCMV negative control plasmid (pGF2-mCMV-rFluc-T2A-GFP-mPGK-Puro) | 10 µg | $655 |
|
||||
| TR411VA-P | pGreenFire 2.0-mCMV negative control virus (pGF2-mCMV-rFluc-T2A-GFP-mPGK-Puro) | >2 x 10^6 IFUs | $655 |
|
||||
Overview
Overview
Make your own pGreenFire 2.0 reporter vector or use as a negative control
With the pGreenFire 2.0 mCMV Cloning & Negative Control Lentivector & Virus (pGF2-mCMV-rFluc-T2A-GFP-mPGK-Puro), you can take advantage of our robust pGreenFire 2.0 lentivector technology to create your own transcriptional response element (TRE) reporter or use pGreenFire 2.0 mCMV as-is as a negative control.
With the pGreenFire 2.0 mCMV Cloning & Negative Control Lentivector, XhoI and NheI sites are placed upstream of a minimal CMV promoter (mCMV) so you can clone in your own TREs. Upon activation, the TREs and mCMV promoter together drive co-expression of red firefly luciferase and GFP so you can quantitatively measure transcriptional activity using both fluorescence and luciferase activity.
Alternatively, you can use the vector as-is as a negative control for any project using pGreenFire 2.0 lentivectors (see data below).
What makes our next-gen pGreenFire 2.0 vectors even better than other TRE reporter vectors is the smart design, which adds in a constitutive selection cassette for stable cell line generation while minimizing interference with the upstream TRE. By using a weak/moderate mPGK promoter to drive the antibiotic selection marker (puromycin resistance) and carefully arranging the conditional reporter genes, the selection marker is reliably expressed without compromising conditional expression of rFLuc and GFP.
As with all of our pGreenFire 2.0 lentivectors, the GreenFire cassette now consists of red firefly luciferase (rFLuc), a T2A co-expression element, and GFP. The switch to rFLuc opens up the possibility of performing a dual-spectral luciferase assay and also delivers greater sensitivity for in vivo applications than conventional luciferase.
References
How It Works
Supporting Data
Supporting Data
See our pGreenFire 2.0 transcriptional response element reporters in action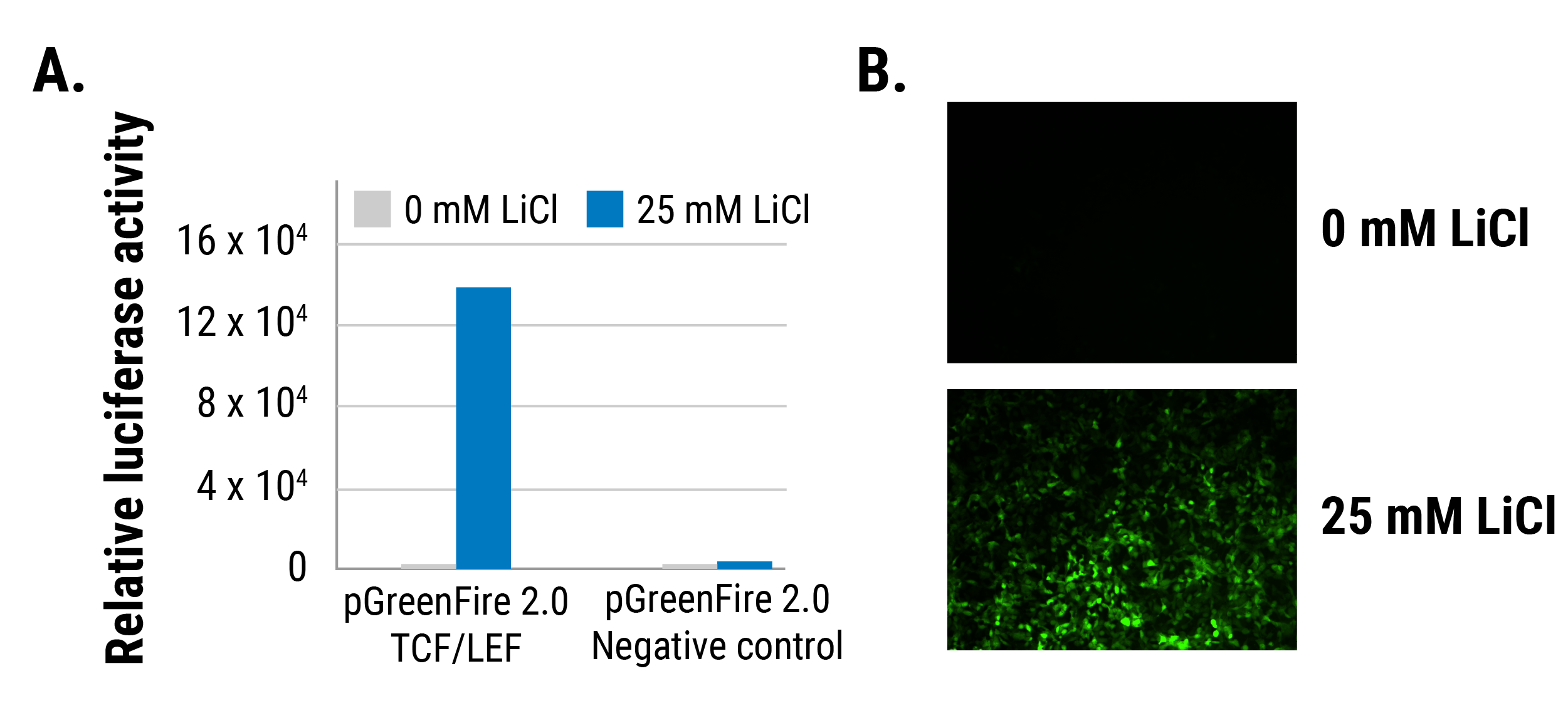
Figure 1. The pGreenFire 2.0 mCMV Negative Control Lentivector delivers negligible expression in 293FT cells—example 1. Compared to induced expression of the pGreenFire 2.0 TCF/LEF lentivector, the pGreenFire 2.0 mCMV Negative Control exhibits virtually no relative luciferase activity (A) and GFP fluorescence (B) regardless of whether the LiCl inducer is used.

Figure 2. The pGreenFire 2.0 mCMV Negative Control Lentivector delivers negligible expression in 293FT cells—example 2. Compared to induced expression of the pGreenFire 2.0 AP-1 lentivector, the pGreenFire 2.0 mCMV Negative Control exhibits virtually no relative luciferase activity (A) and GFP fluorescence (B) regardless of whether the inducer (PMA) is used.
FAQs
Resources
Citations
Related Products
Products
| Catalog Number | Description | Size | Price | Quantity | Add to Cart | |||
|---|---|---|---|---|---|---|---|---|
| TR411PA-P | pGreenFire 2.0-mCMV negative control plasmid (pGF2-mCMV-rFluc-T2A-GFP-mPGK-Puro) | 10 µg | $655 |
|
||||
| TR411VA-P | pGreenFire 2.0-mCMV negative control virus (pGF2-mCMV-rFluc-T2A-GFP-mPGK-Puro) | >2 x 10^6 IFUs | $655 |
|
||||
Overview
Overview
Make your own pGreenFire 2.0 reporter vector or use as a negative control
With the pGreenFire 2.0 mCMV Cloning & Negative Control Lentivector & Virus (pGF2-mCMV-rFluc-T2A-GFP-mPGK-Puro), you can take advantage of our robust pGreenFire 2.0 lentivector technology to create your own transcriptional response element (TRE) reporter or use pGreenFire 2.0 mCMV as-is as a negative control.
With the pGreenFire 2.0 mCMV Cloning & Negative Control Lentivector, XhoI and NheI sites are placed upstream of a minimal CMV promoter (mCMV) so you can clone in your own TREs. Upon activation, the TREs and mCMV promoter together drive co-expression of red firefly luciferase and GFP so you can quantitatively measure transcriptional activity using both fluorescence and luciferase activity.
Alternatively, you can use the vector as-is as a negative control for any project using pGreenFire 2.0 lentivectors (see data below).
What makes our next-gen pGreenFire 2.0 vectors even better than other TRE reporter vectors is the smart design, which adds in a constitutive selection cassette for stable cell line generation while minimizing interference with the upstream TRE. By using a weak/moderate mPGK promoter to drive the antibiotic selection marker (puromycin resistance) and carefully arranging the conditional reporter genes, the selection marker is reliably expressed without compromising conditional expression of rFLuc and GFP.
As with all of our pGreenFire 2.0 lentivectors, the GreenFire cassette now consists of red firefly luciferase (rFLuc), a T2A co-expression element, and GFP. The switch to rFLuc opens up the possibility of performing a dual-spectral luciferase assay and also delivers greater sensitivity for in vivo applications than conventional luciferase.

References
How It Works
Supporting Data
Supporting Data
See our pGreenFire 2.0 transcriptional response element reporters in action
Figure 1. The pGreenFire 2.0 mCMV Negative Control Lentivector delivers negligible expression in 293FT cells—example 1. Compared to induced expression of the pGreenFire 2.0 TCF/LEF lentivector, the pGreenFire 2.0 mCMV Negative Control exhibits virtually no relative luciferase activity (A) and GFP fluorescence (B) regardless of whether the LiCl inducer is used.

Figure 2. The pGreenFire 2.0 mCMV Negative Control Lentivector delivers negligible expression in 293FT cells—example 2. Compared to induced expression of the pGreenFire 2.0 AP-1 lentivector, the pGreenFire 2.0 mCMV Negative Control exhibits virtually no relative luciferase activity (A) and GFP fluorescence (B) regardless of whether the inducer (PMA) is used.

