ExoGlow™-Vivo EV Labeling Kit (Near IR)
- Optimized for imaging EVs in animal models
- High sensitivity—NIR spectrum enables deep tissue illumination and eliminates background from auto-fluorescence
- High specificity—non-lipophilic dye overcomes background from non-specific labeling
- Fast labeling protocol takes you from EV isolation to injection-ready labeled EVs in less than 1 hour
- Ideal for in vivo EV tracking, biodistribution, and kinetic studies
- Assess targeting specificity of engineered EVs
- Follow EVs in organ homing studies
- Compare how different EV isolation methods affect biodistribution and other in vivo parameters critical for evaluating EV performance
- Allows ADME studies of EVs as delivery vehicles
Products
| Catalog Number | Description | Size | Price | Quantity | Add to Cart | |||
|---|---|---|---|---|---|---|---|---|
| EXOGV900A-1 | ExoGlow™-Vivo EV Labeling Kit (Near IR) | 12 Reactions | $589 |
|
||||
Overview
Overview
EV labeling optimized for in vivo imaging Visualizing extracellular vesicles (EVs) in cells and organisms has been technically difficult due to the high background from auto-fluorescence in the UV and visible areas of the spectrum and a lack of specificity from the typically lipophilic dyes. SBI’s ExoGlow-Vivo EV Labeling Kit (Near IR) is the first reagent specifically designed to overcome these problems through the use of a proprietary, non-lipophilic dye that emits in the near infrared (NIR) range (excitation at 784 nm; emission at 806 nm). Delivering a level of specificity and sensitivity that takes the guesswork out of tracking EVs in vivo, ExoGlow-Vivo is ideal for EV biodistribution and kinetic studies needed to fully realize the value of EVs in basic research and translational applications.- Optimized for imaging EVs in animal models
- High sensitivity—NIR spectrum enables deep tissue illumination and eliminates background from auto-fluorescence
- High specificity—non-lipophilic dye overcomes background from non-specific labeling
- Fast labeling protocol takes you from EV isolation to injection-ready labeled EVs in less than 1 hour
- Ideal for in vivo EV tracking, biodistribution, and kinetic studies
- Assess targeting specificity of engineered EVs
- Follow EVs in organ homing studies
- Compare how different EV isolation methods affect biodistribution and other in vivo parameters critical for evaluating EV performance
- Allows ADME studies of EVs as delivery vehicles
- Compatible—delivers robust performance on EVs isolated using all methods tested—including the ExoQuick family, ultracentrifugation, and column-based workflows
- Easy-to-use—labeling protocol is quick and straightforward
- Powerful—can be used with as little as 250 µg of EVs
References
How It Works
Supporting Data
Supporting Data
Imaging EVs with a new level of clarity
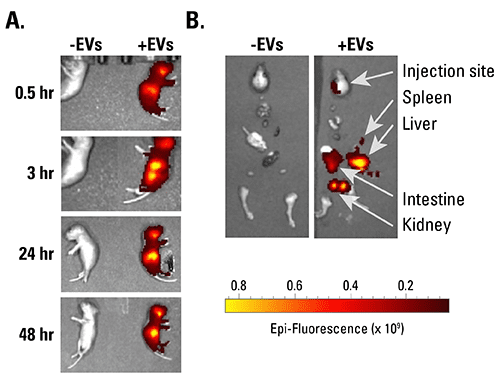
Figure 1. ExoGlow-Vivo labeled EVs show robust signal in vivo. A. HEK293-derived EVs isolated using ExoQuick-TC were labeled with the ExoGlow-Vivo dye and administered intravenously via the superficial temporal vein into post-natal day-4 C57BL6 mice. Animals were imaged at various time points using IVIS® In Vivo Imaging System (PerkinElmer). B. Dissection after 24-hours shows the preferential accumulation of labeled EVs in the liver and kidneys. Data courtesy of Gareth Willis, PhD., Harvard Medical School and Boston Children’s Hospital.
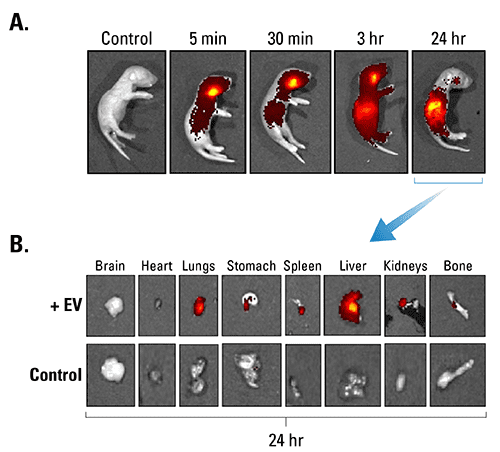
Figure 2. ExoGlow-Vivo dye delivers minimal background. A. Human mesenchymal stem cell-derived EVs were labeled with ExoGlow-Vivo dye and unbound dye removed via ultracentrifugation and a wash. EVs were administered intravenously via the superficial temporal vein into post-natal day-4 FVB mice. Animals were imaged at various time points using an IVIS® In Vivo Imaging System (PerkinElmer). Control refers to supernatant from wash step (i.e. free dye). B. Dissection after 24-hours shows the preferential accumulation of labeled EVs in specific organs and very low residual background signal from free dye. Data courtesy of Gareth Willis, PhD., Harvard Medical School and Boston Children’s Hospital.
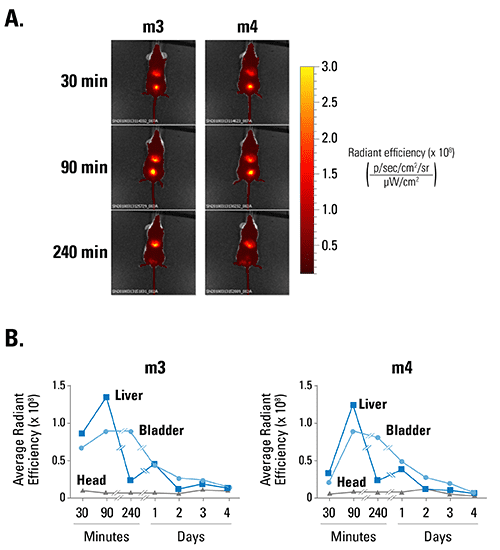
Figure 3. ExoGlow-Vivo enables kinetic analysis of EV persistence in living mice. A. Human PBMC-derived EVs were labeled with ExoGlow-Vivo and administered via tail vein injection into SCID mice (m3 and m4). Animals were imaged at various time points using an IVIS® In Vivo Imaging System (PerkinElmer). B. Plotting signal intensity in different organs (liver, bladder, head) as a function of time after injection shows that EVs are rapidly taken up by target organs within 90-minutes of injection and decline at different rates over time. Data courtesy of Sam Noppen, Rega Institute KU Leuven, Belgium.
FAQs
Documentation
Citations
Related Products
Products
| Catalog Number | Description | Size | Price | Quantity | Add to Cart | |||
|---|---|---|---|---|---|---|---|---|
| EXOGV900A-1 | ExoGlow™-Vivo EV Labeling Kit (Near IR) | 12 Reactions | $589 |
|
||||
Overview
Overview
EV labeling optimized for in vivo imaging Visualizing extracellular vesicles (EVs) in cells and organisms has been technically difficult due to the high background from auto-fluorescence in the UV and visible areas of the spectrum and a lack of specificity from the typically lipophilic dyes. SBI’s ExoGlow-Vivo EV Labeling Kit (Near IR) is the first reagent specifically designed to overcome these problems through the use of a proprietary, non-lipophilic dye that emits in the near infrared (NIR) range (excitation at 784 nm; emission at 806 nm). Delivering a level of specificity and sensitivity that takes the guesswork out of tracking EVs in vivo, ExoGlow-Vivo is ideal for EV biodistribution and kinetic studies needed to fully realize the value of EVs in basic research and translational applications.- Optimized for imaging EVs in animal models
- High sensitivity—NIR spectrum enables deep tissue illumination and eliminates background from auto-fluorescence
- High specificity—non-lipophilic dye overcomes background from non-specific labeling
- Fast labeling protocol takes you from EV isolation to injection-ready labeled EVs in less than 1 hour
- Ideal for in vivo EV tracking, biodistribution, and kinetic studies
- Assess targeting specificity of engineered EVs
- Follow EVs in organ homing studies
- Compare how different EV isolation methods affect biodistribution and other in vivo parameters critical for evaluating EV performance
- Allows ADME studies of EVs as delivery vehicles
- Compatible—delivers robust performance on EVs isolated using all methods tested—including the ExoQuick family, ultracentrifugation, and column-based workflows
- Easy-to-use—labeling protocol is quick and straightforward
- Powerful—can be used with as little as 250 µg of EVs
References
How It Works
Supporting Data
Supporting Data
Imaging EVs with a new level of clarity
Figure 1. ExoGlow-Vivo labeled EVs show robust signal in vivo. A. HEK293-derived EVs isolated using ExoQuick-TC were labeled with the ExoGlow-Vivo dye and administered intravenously via the superficial temporal vein into post-natal day-4 C57BL6 mice. Animals were imaged at various time points using IVIS® In Vivo Imaging System (PerkinElmer). B. Dissection after 24-hours shows the preferential accumulation of labeled EVs in the liver and kidneys. Data courtesy of Gareth Willis, PhD., Harvard Medical School and Boston Children’s Hospital.
Figure 2. ExoGlow-Vivo dye delivers minimal background. A. Human mesenchymal stem cell-derived EVs were labeled with ExoGlow-Vivo dye and unbound dye removed via ultracentrifugation and a wash. EVs were administered intravenously via the superficial temporal vein into post-natal day-4 FVB mice. Animals were imaged at various time points using an IVIS® In Vivo Imaging System (PerkinElmer). Control refers to supernatant from wash step (i.e. free dye). B. Dissection after 24-hours shows the preferential accumulation of labeled EVs in specific organs and very low residual background signal from free dye. Data courtesy of Gareth Willis, PhD., Harvard Medical School and Boston Children’s Hospital.
Figure 3. ExoGlow-Vivo enables kinetic analysis of EV persistence in living mice. A. Human PBMC-derived EVs were labeled with ExoGlow-Vivo and administered via tail vein injection into SCID mice (m3 and m4). Animals were imaged at various time points using an IVIS® In Vivo Imaging System (PerkinElmer). B. Plotting signal intensity in different organs (liver, bladder, head) as a function of time after injection shows that EVs are rapidly taken up by target organs within 90-minutes of injection and decline at different rates over time. Data courtesy of Sam Noppen, Rega Institute KU Leuven, Belgium.