CAG-T7-hspCas9-Nickase-T2A-RFP-H1-gRNA All-in-one Cas9 SmartNickase™ Plasmid
- Conveniently deliver Cas9 SmartNickase and gRNA with a single vector
- Reduce off-target activity with Cas9 SmartNickase
- Monitor transfection efficiencies with RFP, which is coordinately expressed with the hspCas9 gene via the T2A element
- Drive Cas9 expression with the CAG promoter, which provides high expression levels in primary cells and stem cells
- Express gRNA from the H1 promoter for maximum specificity and choice of targets
Products
| Catalog Number | Description | Size | Price | Quantity | Add to Cart | |||
|---|---|---|---|---|---|---|---|---|
| CAS771R-1 | All-in-one Cas9 Nickase: CAG-T7-hspCas9-nickase-T2A-RFP-H1-gRNA linearized SmartNickase vector | 10 Reactions | $819 |
|
||||
Overview
Overview
Conveniently check transfection efficiencies with this All-in-one Cas9 SmartNickase
Our All-in-one Cas9 and gRNA plasmids are an excellent way to simplify delivery of your CRISPR/Cas9 Nickase system by providing both Cas9 Nickase and gRNA from a single vector, and the addition of coordinate expression of RFP for monitoring transfection efficiencies helps make genome engineering projects more user-friendly. SBI’s CAG-T7-hspCas9-Nickase-T2A-RFP-H1-gRNA All-in-one Cas9 SmartNickase Plasmid includes a number of additional features that make it a great All-in-one choice for any genome engineering project involving transfectable cells:
- Conveniently deliver Cas9 SmartNickase and gRNA with a single vector
- Reduce off-target activity with Cas9 SmartNickase
- Monitor transfection efficiencies with RFP, which is coordinately expressed with the hspCas9 gene via the T2A element
- Drive Cas9 expression with the CAG promoter, which provides high expression levels in primary cells and stem cells
- Express gRNA from the H1 promoter for maximum specificity and choice of targets
- Ensure efficient import of Cas9 to the nucleus with N-term and C-term nuclear localization signals (NLSs)
- Boost Cas9 gene expression and stabilize the transcript via the WPRE regulatory element after the C-term NLS
- Easily detect and/or purify the Cas9 protein with the N-term myc-tag
- Produce Cas9 mRNA via in vitro transcription using the T7 promoter
As with all of our Cas9 delivery options, the CAG-T7-hspCas9-Nickase-T2A-RFP-H1-gRNA Plasmid is functionally validated and comes backed by our expert technical support team—if you’ve got a genome engineering question just ask by emailing tech@systembio.com.
Why an HR targeting vector is a recommended
Even though gene knock-outs can result from DSBs caused by Cas9 alone, SBI recommends the use of HR targeting vectors (also called HR donor vectors) for more efficient and precise mutation. HR donors can supply elements for positive or negative selection ensuring easier identification of successful mutation events. In addition, HR donors can include up to 6-8 kb of open reading frame for gene knock-ins or tagging, and, when small mutations are included in either 5’ or 3’ homology arms, can make specific, targeted gene edits.
Not sure whether you need a CRISPR/Cas9 plasmid, purified protein, or mRNA?
Use this table to choose the CRISPR/Cas9 product that’s right for you:
For This Application | In these types of cells | Use These Products |
|---|---|---|
MODIFYING ORGANISMS
| Embryos—to create transgenic animals | Injectable Cas9 mRNA & gRNA Synthesis Kits Cas9 Protein EGFP-labeled Cas9 Protein |
| Animals models—in vivo genome editing | AAV-Cas9 Vectors Cas9 Protein EGFP-labeled Cas9 Protein |
|
MODIFYING CELL LINES
| Cells that are transfectable | Cas9 Plasmids Cas9 Protein EGFP-labeled Cas9 Protein |
Difficult-to-transfect cell lines:
| AAV-Cas9 Vectors Lenti Cas9 Systems |
|
SCREENING
| All cell types requiring stable Cas9 overexpression | Lenti Cas9 Systems AAVS1 Safe Harbor Site Cas9 Gene Knock-in System Cas9 Protein EGFP-labeled Cas9 Protein |
PRE-CLINICAL APPLICATIONS
| All cell types and applications | Cas9 Nickase, available in all delivery formats Cas9 Protein EGFP-labeled Cas9 Protein |
| SIMULTANEOUS ENGINEERING OF MULTIPLE MUTATIONS | All cell types and applications | Multiplex gRNA cloning kit, compatible with all Cas9 delivery options |
References
How It Works
How It Works
Genome engineering with CRISPR/Cas9
For general guidance on using CRISPR/Cas9 technology for genome engineering, take a look at our CRISPR/Cas9 tutorials as well as the following application notes:
CRISPR/Cas9 Gene Knock-Out Application Note (PDF) »
CRISPR/Cas9 Gene Editing Application Note (PDF) »
CRISPR/Cas9 Gene Tagging Application Note (PDF) »
CRISPR/Cas9 Basics
Through careful selection of the target sequence and design of a donor plasmid for homologous recombination, you can achieve efficient and highly targeted genomic modification with CRISPR/Cas9.
The system
Cas9 protein—uses guide RNA (gRNA) to direct site-specific, double-strand DNA cleavage adjacent to a protospacer adapter motif (PAM) in the target DNA.
gRNA—RNA sequence that guides Cas9 to cleave a homologous region in the target genome. Efficient cleavage only where the gRNA homology is adjacent to a PAM.
PAM—protospacer adapter motif, NGG, is a target DNA sequence that spCas9 will cut upstream from if directed to by the gRNA.
The workflow at-a-glance
DESIGN: Select gRNA and HR donor plasmids. Choice of gRNA site and design of donor plasmid determines whether the homologous recombination event results in a knock-out, knock-in, edit, or tagging.
CONSTRUCT: Clone gRNA into all-in-one Cas9 vector. Clone 5’ and 3’ homology arms into HR donor plasmid. If creating a knock-in, clone desired gene into HR donor.
CO-TRANSFECT or CO-INJECT: Introduce Cas9, gRNA, and HR Donors into the target cells using co-transfection for plasmids, co-transduction for lentivirus, or co-injection for mRNAs.
SELECT/SCREEN: Select or screen for mutants and verify.
VALIDATE: Genotype or sequence putative mutants to verify single or biallelic conversion.
Supporting Data
Supporting Data
Simultaneous editing at multiple genomic locations
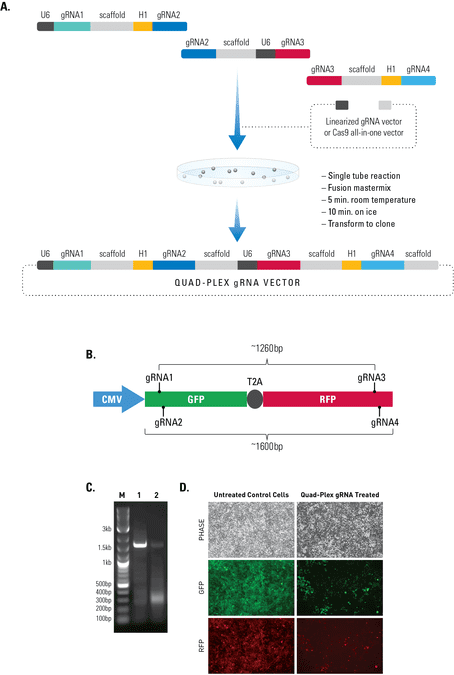
Figure 2. The Multiplex gRNA Cloning Kit enables efficient delivery of four gRNAs.(A) We used a quad-plex gRNA created using the Multilplex gRNA Cloning Kit and an All-in-one Cas9 SmartNickase Vector to remove a (B) 1260 bp GFP-T2A-RFP segment from a cell line with a stably integrated CMV-GFP-T2A-RFP expression cassette. We cloned the four gRNAs into a Cas9 SmartNickase vector (EF1Nickase-H1-gRNA) to guide two double nicking events—one at the 5’ end of the GFP and the other at the 3’ end of the RFP gene. (C) PCR assays with primers just outside of the GFP and RFP genes generate a 1600 bp fragment in the absence of the SmartNickase vector (lane 1), and a 340 bp fragment in the presence of the Cas9 SmartNickase-4 gRNA construct (lane 2), demonstrating the efficiency of SmartNickase-mediated paired double-nicking and GFP-T2A-RFP genomic deletion. (D) Deletion of both GFP and RFP activities can also be seen in a functional assay, through reduction in both GFP and RFP fluorescence.
FAQs
Documentation
Citations
Related Products
Products
| Catalog Number | Description | Size | Price | Quantity | Add to Cart | |||
|---|---|---|---|---|---|---|---|---|
| CAS771R-1 | All-in-one Cas9 Nickase: CAG-T7-hspCas9-nickase-T2A-RFP-H1-gRNA linearized SmartNickase vector | 10 Reactions | $819 |
|
||||
Overview
Overview
Conveniently check transfection efficiencies with this All-in-one Cas9 SmartNickase
Our All-in-one Cas9 and gRNA plasmids are an excellent way to simplify delivery of your CRISPR/Cas9 Nickase system by providing both Cas9 Nickase and gRNA from a single vector, and the addition of coordinate expression of RFP for monitoring transfection efficiencies helps make genome engineering projects more user-friendly. SBI’s CAG-T7-hspCas9-Nickase-T2A-RFP-H1-gRNA All-in-one Cas9 SmartNickase Plasmid includes a number of additional features that make it a great All-in-one choice for any genome engineering project involving transfectable cells:
- Conveniently deliver Cas9 SmartNickase and gRNA with a single vector
- Reduce off-target activity with Cas9 SmartNickase
- Monitor transfection efficiencies with RFP, which is coordinately expressed with the hspCas9 gene via the T2A element
- Drive Cas9 expression with the CAG promoter, which provides high expression levels in primary cells and stem cells
- Express gRNA from the H1 promoter for maximum specificity and choice of targets
- Ensure efficient import of Cas9 to the nucleus with N-term and C-term nuclear localization signals (NLSs)
- Boost Cas9 gene expression and stabilize the transcript via the WPRE regulatory element after the C-term NLS
- Easily detect and/or purify the Cas9 protein with the N-term myc-tag
- Produce Cas9 mRNA via in vitro transcription using the T7 promoter
As with all of our Cas9 delivery options, the CAG-T7-hspCas9-Nickase-T2A-RFP-H1-gRNA Plasmid is functionally validated and comes backed by our expert technical support team—if you’ve got a genome engineering question just ask by emailing tech@systembio.com.
Why an HR targeting vector is a recommended
Even though gene knock-outs can result from DSBs caused by Cas9 alone, SBI recommends the use of HR targeting vectors (also called HR donor vectors) for more efficient and precise mutation. HR donors can supply elements for positive or negative selection ensuring easier identification of successful mutation events. In addition, HR donors can include up to 6-8 kb of open reading frame for gene knock-ins or tagging, and, when small mutations are included in either 5’ or 3’ homology arms, can make specific, targeted gene edits.
Not sure whether you need a CRISPR/Cas9 plasmid, purified protein, or mRNA?
Use this table to choose the CRISPR/Cas9 product that’s right for you:
For This Application | In these types of cells | Use These Products |
|---|---|---|
MODIFYING ORGANISMS
| Embryos—to create transgenic animals | Injectable Cas9 mRNA & gRNA Synthesis Kits Cas9 Protein EGFP-labeled Cas9 Protein |
| Animals models—in vivo genome editing | AAV-Cas9 Vectors Cas9 Protein EGFP-labeled Cas9 Protein |
|
MODIFYING CELL LINES
| Cells that are transfectable | Cas9 Plasmids Cas9 Protein EGFP-labeled Cas9 Protein |
Difficult-to-transfect cell lines:
| AAV-Cas9 Vectors Lenti Cas9 Systems |
|
SCREENING
| All cell types requiring stable Cas9 overexpression | Lenti Cas9 Systems AAVS1 Safe Harbor Site Cas9 Gene Knock-in System Cas9 Protein EGFP-labeled Cas9 Protein |
PRE-CLINICAL APPLICATIONS
| All cell types and applications | Cas9 Nickase, available in all delivery formats Cas9 Protein EGFP-labeled Cas9 Protein |
| SIMULTANEOUS ENGINEERING OF MULTIPLE MUTATIONS | All cell types and applications | Multiplex gRNA cloning kit, compatible with all Cas9 delivery options |
References
How It Works
How It Works
Genome engineering with CRISPR/Cas9
For general guidance on using CRISPR/Cas9 technology for genome engineering, take a look at our CRISPR/Cas9 tutorials as well as the following application notes:
CRISPR/Cas9 Gene Knock-Out Application Note (PDF) »
CRISPR/Cas9 Gene Editing Application Note (PDF) »
CRISPR/Cas9 Gene Tagging Application Note (PDF) »
CRISPR/Cas9 Basics
Through careful selection of the target sequence and design of a donor plasmid for homologous recombination, you can achieve efficient and highly targeted genomic modification with CRISPR/Cas9.
The system
Cas9 protein—uses guide RNA (gRNA) to direct site-specific, double-strand DNA cleavage adjacent to a protospacer adapter motif (PAM) in the target DNA.
gRNA—RNA sequence that guides Cas9 to cleave a homologous region in the target genome. Efficient cleavage only where the gRNA homology is adjacent to a PAM.
PAM—protospacer adapter motif, NGG, is a target DNA sequence that spCas9 will cut upstream from if directed to by the gRNA.
The workflow at-a-glance
DESIGN: Select gRNA and HR donor plasmids. Choice of gRNA site and design of donor plasmid determines whether the homologous recombination event results in a knock-out, knock-in, edit, or tagging.
CONSTRUCT: Clone gRNA into all-in-one Cas9 vector. Clone 5’ and 3’ homology arms into HR donor plasmid. If creating a knock-in, clone desired gene into HR donor.
CO-TRANSFECT or CO-INJECT: Introduce Cas9, gRNA, and HR Donors into the target cells using co-transfection for plasmids, co-transduction for lentivirus, or co-injection for mRNAs.
SELECT/SCREEN: Select or screen for mutants and verify.
VALIDATE: Genotype or sequence putative mutants to verify single or biallelic conversion.
Supporting Data
Supporting Data
Simultaneous editing at multiple genomic locations
Figure 2. The Multiplex gRNA Cloning Kit enables efficient delivery of four gRNAs.(A) We used a quad-plex gRNA created using the Multilplex gRNA Cloning Kit and an All-in-one Cas9 SmartNickase Vector to remove a (B) 1260 bp GFP-T2A-RFP segment from a cell line with a stably integrated CMV-GFP-T2A-RFP expression cassette. We cloned the four gRNAs into a Cas9 SmartNickase vector (EF1Nickase-H1-gRNA) to guide two double nicking events—one at the 5’ end of the GFP and the other at the 3’ end of the RFP gene. (C) PCR assays with primers just outside of the GFP and RFP genes generate a 1600 bp fragment in the absence of the SmartNickase vector (lane 1), and a 340 bp fragment in the presence of the Cas9 SmartNickase-4 gRNA construct (lane 2), demonstrating the efficiency of SmartNickase-mediated paired double-nicking and GFP-T2A-RFP genomic deletion. (D) Deletion of both GFP and RFP activities can also be seen in a functional assay, through reduction in both GFP and RFP fluorescence.