Cas9 Protein & T7 gRNA SmartNuclease™ Synthesis Kit
- Highly concentrated and ready to use—no reconstitution required
- Acts immediately upon entry into the cell
- Rapid clearance reduces chances for off-target cleavage events
- Avoids issues with genomic integration
- Enables in-vitro cleavage assays
Products
| Catalog Number | Description | Size | Price | Quantity | Add to Cart | |||
|---|---|---|---|---|---|---|---|---|
| CAS420A-KIT | EGFP-labeled Cas9 Protein (NLS-Cas9-EGFP) and T7 gRNA SmartNuclease Synthesis Kit | 1 Kit (10 Reactions) | $1235 |
|
||||
| CAS410A-KIT | Purified Cas9 Protein (NLS-Cas9-NLS) and T7 gRNA SmartNuclease Synthesis Kit | 1 Kit (10 Reactions) | $1193 |
|
||||
Overview
Overview
Get Purified Cas9 Protein bundled with gRNA production
Combining a T7 gRNA SmartNuclease™ Synthesis Kit with purified, transfection-/electroporation-ready Cas9 Protein or EGFP-labeled Cas9 Protein gives you everything you need for targeted genome editing (note that many applications including gene knock-outs, knock-ins, and edits also require the use of a homologous recombination (HR) targeting vector—see our CRISPR/Cas9 tutorials to learn more).
Cas9 Protein & T7 gRNA SmartNuclease™ Synthesis Kit includes Purified Cas9 Protein (NLS-Cas9-NLS, Cat.# CAS410A-1) or EGFP-labeled Cas 9 Protein (NLS-Cas9-EGFP, Cat.# CAS420A-1) and the T7 gRNA SmartNuclease Synthesis Kit (Cat.# CAS510A-KIT).
Taking CRISPR/Cas9 technology to the next level
With the Cas9 protein, you can get more efficient genome editing while reducing off-target events1–3. By removing plasmid delivery of Cas9 from the genome editing process, both off-target events from random plasmid integration and the potential for an immune response from bacterial plasmid sequences are avoided1,2. In addition, the more transient nature of Cas9 protein compared to plasmid or mRNA delivery further reduces off-target activity without decreasing on-target efficiency1,2. The end result is better, safer Cas9 activity for applications where off-target events need to be minimized, such as:
- Genome engineering in embryos
- Disease model generation of organisms and cell lines
- In vitro transfection of cells
- In vitro cleavage assays for functional gRNA screens
- Increase Cas9 efficiency2
- Reduce off-target events2
- Reduce the potential for immune response to vector DNA
- Simplify delivery to cells and embryos
- Perform multiplex, high-throughput studies
- Conduct typical downstream functional assays
Efficiently generate gRNA with the T7 gRNA SmartNuclease Synthesis Kit
The T7 gRNA SmartNuclease Synthesis Kit comes with a pre-linearized, ready-for-cloning gRNA expression vector (Figure 1) and all the reagents you need for T7-driven in vitro transcription of your cloned gRNA.

Figure 1. The T7 gRNA SmartNuclease Cloning and Production Vector.
Not sure whether you need a CRISPR/Cas9 plasmid, purified protein, or mRNA?
Use this table to choose the CRISPR/Cas9 product that’s right for you:
For This Application | In these types of cells | Use These Products |
|---|---|---|
MODIFYING ORGANISMS
| Embryos—to create transgenic animals | Injectable Cas9 mRNA & gRNA Synthesis Kits Cas9 Protein EGFP-labeled Cas9 Protein |
| Animals models—in vivo genome editing | AAV-Cas9 Vectors Cas9 Protein EGFP-labeled Cas9 Protein |
|
MODIFYING CELL LINES
| Cells that are transfectable | Cas9 Plasmids Cas9 Protein EGFP-labeled Cas9 Protein |
Difficult-to-transfect cell lines:
| AAV-Cas9 Vectors Lenti Cas9 Systems |
|
SCREENING
| All cell types requiring stable Cas9 overexpression | Lenti Cas9 Systems AAVS1 Safe Harbor Site Cas9 Gene Knock-in System Cas9 Protein EGFP-labeled Cas9 Protein |
PRE-CLINICAL APPLICATIONS
| All cell types and applications | Cas9 Nickase, available in all delivery formats Cas9 Protein EGFP-labeled Cas9 Protein |
| SIMULTANEOUS ENGINEERING OF MULTIPLE MUTATIONS | All cell types and applications | Multiplex gRNA cloning kit, compatible with all Cas9 delivery options |
References
- Ramakrishna, S. et al. Gene disruption by cell-penetrating peptide-mediated delivery of Cas9 protein and guide RNA. Genome Res. (2014). 24:1020–1027. PMCID: PMC4032848.
- Wang, L. et al. Large genomic fragment deletion and functional gene cassette knock-in via Cas9 protein mediated genome editing in one-cell rodent embryos. Sci. Rep. (2015). 5:17517. PMCID: PMC4664917.
- Chen, S., et al. Highly Efficient Mouse Genome Editing by CRISPR Ribonucleoprotein Electroporation of Zygotes. J. Biol. Chem. (2016). 291(28):14457-67. PMID: 27151215.
References
How It Works
How It Works
Using Transfectable/Electroporatable Cas9 Protein
Using SBI’s transfectable or electroporatable Cas9 protein/EGFP-labeled Cas9 Protein is quick and easy. Simply pre-incubate Cas9 protein with your gRNA and then either transfect or electroporate as normal. While a 1:1 ratio of Cas9 protein to gRNA was used for the study in the Supporting Data section below, we recommend optimizing the amounts and ratios for your specific gRNA and cell lines.
For more general guidance on using CRISPR/Cas9 technology for genome engineering, take a look at our CRISPR/Cas9 tutorials as well as the following application notes:
CRISPR/Cas9 Gene Knock-Out Application Note (PDF) »
CRISPR/Cas9 Gene Editing Application Note (PDF) »
CRISPR/Cas9 Gene Tagging Application Note (PDF) »
CRISPR/Cas9 Basics
Through careful selection of the target sequence and design of a donor plasmid for homologous recombination, you can achieve efficient and highly targeted genomic modification with CRISPR/Cas9.
The system

Figure 2. The Cas9 system.
Cas9 protein—uses guide RNA (gRNA) to direct site-specific, double-strand DNA cleavage adjacent to a protospacer adapter motif (PAM) in the target DNA.
gRNA—RNA sequence that guides Cas9 to cleave a homologous region in the target genome. Efficient cleavage only where the gRNA homology is adjacent to a PAM.
PAM—protospacer adapter motif, NGG, is a target DNA sequence that spCas9 will cut upstream from if directed to by the gRNA.
The workflow at-a-glance
DESIGN: Select gRNA and HR donor plasmids. Choice of gRNA site and design of donor plasmid determines whether the homologous recombination event results in a knock-out, knock-in, edit, or tagging.
CONSTRUCT: Clone gRNA into all-in-one Cas9 vector. Clone 5’ and 3’ homology arms into HR donor plasmid. If creating a knock-in, clone desired gene into HR donor.
CO-TRANSFECT or CO-INJECT: Introduce Cas9, gRNA, and HR Donors into the target cells using co-transfection for plasmids, co-transduction for lentivirus, or co-injection for mRNAs.
SELECT/SCREEN: Select or screen for mutants and verify.
VALIDATE: Genotype or sequence putative mutants to verify single or biallelic conversion.
Supporting Data
Supporting Data
NLS-Cas9-NLS protein is functional in an in vivo assay as seen in a DNA gel
To demonstrate that the NLS-Cas9-NLS protein possesses similar levels of activity as Cas9 introduced using a lentivector—our Cas9 All-in-one System—we created a gRNA that generates a one nt change in the endogenous miR-21 gene, delivered NLS-Cas9-EGFP using either CRISPRMax or cell-penetrating peptide (CPP), and performed a T7E1 mismatch detection assay (Figure 3). Levels of the cleavage product in cells with NLS-Cas9-NLS are similar to the levels generated using our Cas9 All-in-one System, demonstrating the activity of the protein.
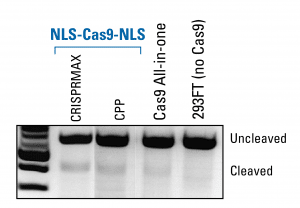
Figure 3. NLS-Cas9-NLS protein is functional in an in vivo assay as seen in a DNA gel.
NLS-Cas9-EGFP can be transfected and electroporated into cells
NLS-Cas9-EGFP can be introduced into cells by transfection—using CRISPRMAX and a cell-penetrating peptide system (CPP)—as well by electroporation (Figure 4).
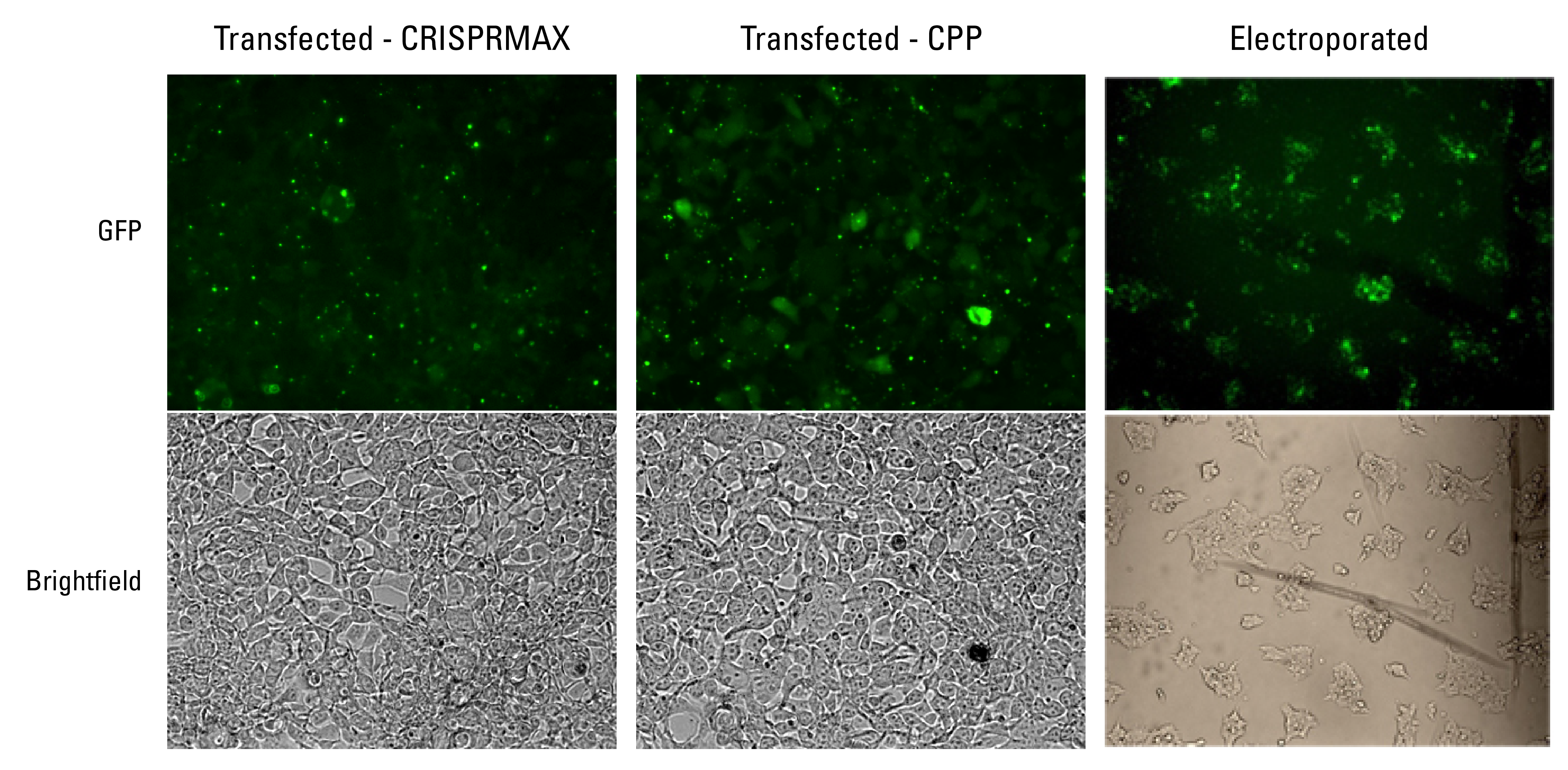
Figure 4. NLS-Cas9-EGFP can be transfected and electroporated into cells
NLS-Cas9-EGFP protein is functional in an in vitro assay
NLS-Cas9-EGFP can be used for in vitro studies (Figure 5). We linearized pUC57 plasmid DNA with EcoRV and assessed nuclease activity and specificity of NLS-Cas9-EGFP by appearance of a cleavage product. Lanes with gRNA and NLS-Cas9-EGFP exhibit the expected cleavage products in a dosage-dependent manner, demonstrating the in vitro activity and specificity of NLS-Cas9-EGFP.
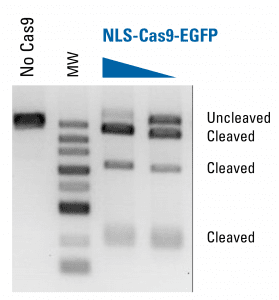
Figure 5. NLS-Cas9-EGFP protein is functional in an in vitro assay.
NLS-Cas9-EGFP protein is functional in an in vivo assay
To demonstrate that the NLS-Cas9-EGFP protein possesses similar levels of activity as Cas9 introduced using a lentivector—our Cas9 All-in-one System—we created a gRNA that generates a one nt change in the endogenous miR-21 gene, delivered NLS-Cas9-EGFP using either CRISPRMax or cell-penetrating peptide (CPP), and performed a T7E1 mismatch detection assay (Figure 6A). Both methods of introducing NLS-Cas9-EGFP result in a cleavage product, demonstrating the protein’s activity. Levels of the cleavage product are similar to the levels generated using our Cas9 All-in-one System, and no activity is seen in the absence of Cas9. Figure 6B shows a similar set of studies for NLS-Cas9-EGFP electroporated into target cells.
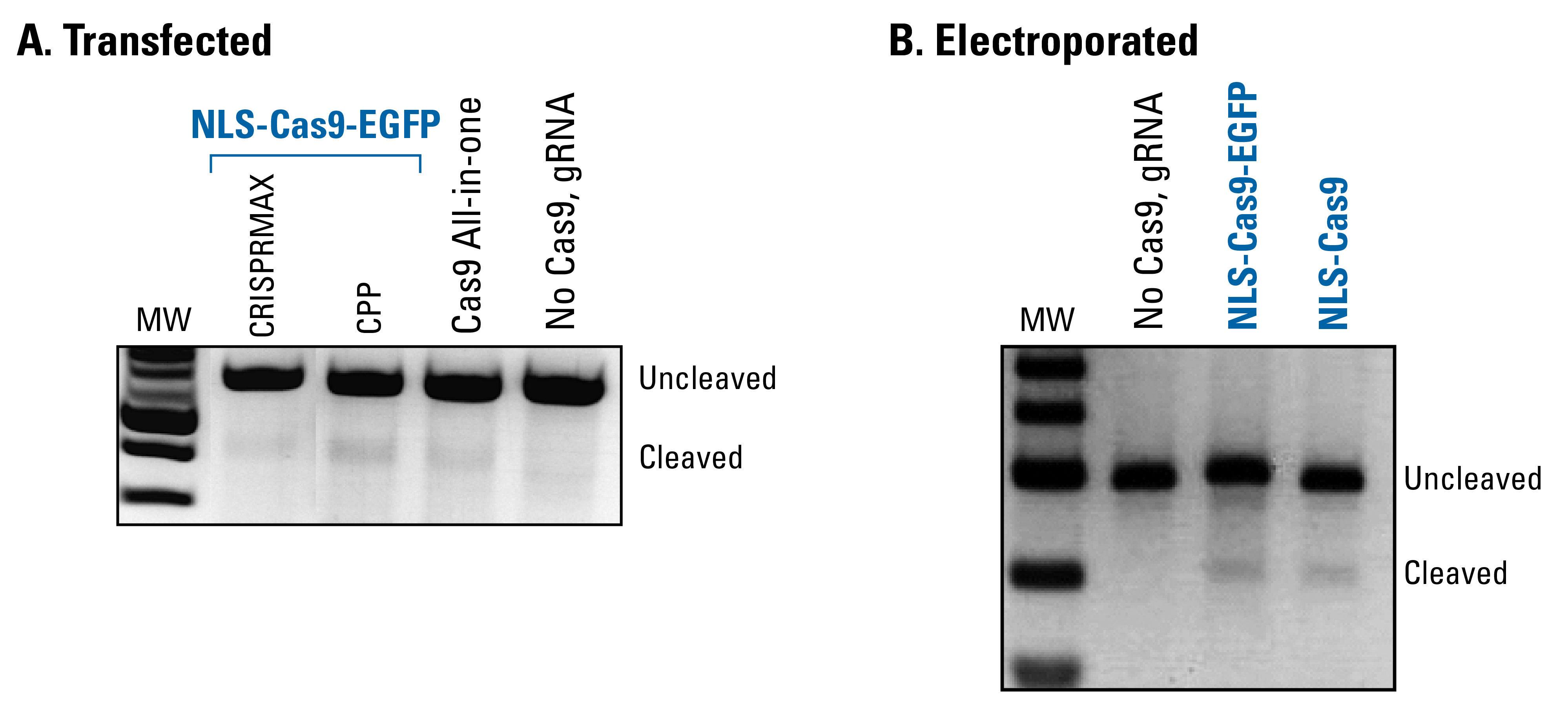
Figure 6. NLS-Cas9-EGFP protein is functional in an in vivo assay.
FAQs
Documentation
Citations
Related Products
Products
| Catalog Number | Description | Size | Price | Quantity | Add to Cart | |||
|---|---|---|---|---|---|---|---|---|
| CAS420A-KIT | EGFP-labeled Cas9 Protein (NLS-Cas9-EGFP) and T7 gRNA SmartNuclease Synthesis Kit | 1 Kit (10 Reactions) | $1235 |
|
||||
| CAS410A-KIT | Purified Cas9 Protein (NLS-Cas9-NLS) and T7 gRNA SmartNuclease Synthesis Kit | 1 Kit (10 Reactions) | $1193 |
|
||||
Overview
Overview
Get Purified Cas9 Protein bundled with gRNA production
Combining a T7 gRNA SmartNuclease™ Synthesis Kit with purified, transfection-/electroporation-ready Cas9 Protein or EGFP-labeled Cas9 Protein gives you everything you need for targeted genome editing (note that many applications including gene knock-outs, knock-ins, and edits also require the use of a homologous recombination (HR) targeting vector—see our CRISPR/Cas9 tutorials to learn more).
Cas9 Protein & T7 gRNA SmartNuclease™ Synthesis Kit includes Purified Cas9 Protein (NLS-Cas9-NLS, Cat.# CAS410A-1) or EGFP-labeled Cas 9 Protein (NLS-Cas9-EGFP, Cat.# CAS420A-1) and the T7 gRNA SmartNuclease Synthesis Kit (Cat.# CAS510A-KIT).
Taking CRISPR/Cas9 technology to the next level
With the Cas9 protein, you can get more efficient genome editing while reducing off-target events1–3. By removing plasmid delivery of Cas9 from the genome editing process, both off-target events from random plasmid integration and the potential for an immune response from bacterial plasmid sequences are avoided1,2. In addition, the more transient nature of Cas9 protein compared to plasmid or mRNA delivery further reduces off-target activity without decreasing on-target efficiency1,2. The end result is better, safer Cas9 activity for applications where off-target events need to be minimized, such as:
- Genome engineering in embryos
- Disease model generation of organisms and cell lines
- In vitro transfection of cells
- In vitro cleavage assays for functional gRNA screens
- Increase Cas9 efficiency2
- Reduce off-target events2
- Reduce the potential for immune response to vector DNA
- Simplify delivery to cells and embryos
- Perform multiplex, high-throughput studies
- Conduct typical downstream functional assays
Efficiently generate gRNA with the T7 gRNA SmartNuclease Synthesis Kit
The T7 gRNA SmartNuclease Synthesis Kit comes with a pre-linearized, ready-for-cloning gRNA expression vector (Figure 1) and all the reagents you need for T7-driven in vitro transcription of your cloned gRNA.

Figure 1. The T7 gRNA SmartNuclease Cloning and Production Vector.
Not sure whether you need a CRISPR/Cas9 plasmid, purified protein, or mRNA?
Use this table to choose the CRISPR/Cas9 product that’s right for you:
For This Application | In these types of cells | Use These Products |
|---|---|---|
MODIFYING ORGANISMS
| Embryos—to create transgenic animals | Injectable Cas9 mRNA & gRNA Synthesis Kits Cas9 Protein EGFP-labeled Cas9 Protein |
| Animals models—in vivo genome editing | AAV-Cas9 Vectors Cas9 Protein EGFP-labeled Cas9 Protein |
|
MODIFYING CELL LINES
| Cells that are transfectable | Cas9 Plasmids Cas9 Protein EGFP-labeled Cas9 Protein |
Difficult-to-transfect cell lines:
| AAV-Cas9 Vectors Lenti Cas9 Systems |
|
SCREENING
| All cell types requiring stable Cas9 overexpression | Lenti Cas9 Systems AAVS1 Safe Harbor Site Cas9 Gene Knock-in System Cas9 Protein EGFP-labeled Cas9 Protein |
PRE-CLINICAL APPLICATIONS
| All cell types and applications | Cas9 Nickase, available in all delivery formats Cas9 Protein EGFP-labeled Cas9 Protein |
| SIMULTANEOUS ENGINEERING OF MULTIPLE MUTATIONS | All cell types and applications | Multiplex gRNA cloning kit, compatible with all Cas9 delivery options |
References
- Ramakrishna, S. et al. Gene disruption by cell-penetrating peptide-mediated delivery of Cas9 protein and guide RNA. Genome Res. (2014). 24:1020–1027. PMCID: PMC4032848.
- Wang, L. et al. Large genomic fragment deletion and functional gene cassette knock-in via Cas9 protein mediated genome editing in one-cell rodent embryos. Sci. Rep. (2015). 5:17517. PMCID: PMC4664917.
- Chen, S., et al. Highly Efficient Mouse Genome Editing by CRISPR Ribonucleoprotein Electroporation of Zygotes. J. Biol. Chem. (2016). 291(28):14457-67. PMID: 27151215.
References
How It Works
How It Works
Using Transfectable/Electroporatable Cas9 Protein
Using SBI’s transfectable or electroporatable Cas9 protein/EGFP-labeled Cas9 Protein is quick and easy. Simply pre-incubate Cas9 protein with your gRNA and then either transfect or electroporate as normal. While a 1:1 ratio of Cas9 protein to gRNA was used for the study in the Supporting Data section below, we recommend optimizing the amounts and ratios for your specific gRNA and cell lines.
For more general guidance on using CRISPR/Cas9 technology for genome engineering, take a look at our CRISPR/Cas9 tutorials as well as the following application notes:
CRISPR/Cas9 Gene Knock-Out Application Note (PDF) »
CRISPR/Cas9 Gene Editing Application Note (PDF) »
CRISPR/Cas9 Gene Tagging Application Note (PDF) »
CRISPR/Cas9 Basics
Through careful selection of the target sequence and design of a donor plasmid for homologous recombination, you can achieve efficient and highly targeted genomic modification with CRISPR/Cas9.
The system

Figure 2. The Cas9 system.
Cas9 protein—uses guide RNA (gRNA) to direct site-specific, double-strand DNA cleavage adjacent to a protospacer adapter motif (PAM) in the target DNA.
gRNA—RNA sequence that guides Cas9 to cleave a homologous region in the target genome. Efficient cleavage only where the gRNA homology is adjacent to a PAM.
PAM—protospacer adapter motif, NGG, is a target DNA sequence that spCas9 will cut upstream from if directed to by the gRNA.
The workflow at-a-glance
DESIGN: Select gRNA and HR donor plasmids. Choice of gRNA site and design of donor plasmid determines whether the homologous recombination event results in a knock-out, knock-in, edit, or tagging.
CONSTRUCT: Clone gRNA into all-in-one Cas9 vector. Clone 5’ and 3’ homology arms into HR donor plasmid. If creating a knock-in, clone desired gene into HR donor.
CO-TRANSFECT or CO-INJECT: Introduce Cas9, gRNA, and HR Donors into the target cells using co-transfection for plasmids, co-transduction for lentivirus, or co-injection for mRNAs.
SELECT/SCREEN: Select or screen for mutants and verify.
VALIDATE: Genotype or sequence putative mutants to verify single or biallelic conversion.
Supporting Data
Supporting Data
NLS-Cas9-NLS protein is functional in an in vivo assay as seen in a DNA gel
To demonstrate that the NLS-Cas9-NLS protein possesses similar levels of activity as Cas9 introduced using a lentivector—our Cas9 All-in-one System—we created a gRNA that generates a one nt change in the endogenous miR-21 gene, delivered NLS-Cas9-EGFP using either CRISPRMax or cell-penetrating peptide (CPP), and performed a T7E1 mismatch detection assay (Figure 3). Levels of the cleavage product in cells with NLS-Cas9-NLS are similar to the levels generated using our Cas9 All-in-one System, demonstrating the activity of the protein.

Figure 3. NLS-Cas9-NLS protein is functional in an in vivo assay as seen in a DNA gel.
NLS-Cas9-EGFP can be transfected and electroporated into cells
NLS-Cas9-EGFP can be introduced into cells by transfection—using CRISPRMAX and a cell-penetrating peptide system (CPP)—as well by electroporation (Figure 4).

Figure 4. NLS-Cas9-EGFP can be transfected and electroporated into cells
NLS-Cas9-EGFP protein is functional in an in vitro assay
NLS-Cas9-EGFP can be used for in vitro studies (Figure 5). We linearized pUC57 plasmid DNA with EcoRV and assessed nuclease activity and specificity of NLS-Cas9-EGFP by appearance of a cleavage product. Lanes with gRNA and NLS-Cas9-EGFP exhibit the expected cleavage products in a dosage-dependent manner, demonstrating the in vitro activity and specificity of NLS-Cas9-EGFP.

Figure 5. NLS-Cas9-EGFP protein is functional in an in vitro assay.
NLS-Cas9-EGFP protein is functional in an in vivo assay
To demonstrate that the NLS-Cas9-EGFP protein possesses similar levels of activity as Cas9 introduced using a lentivector—our Cas9 All-in-one System—we created a gRNA that generates a one nt change in the endogenous miR-21 gene, delivered NLS-Cas9-EGFP using either CRISPRMax or cell-penetrating peptide (CPP), and performed a T7E1 mismatch detection assay (Figure 6A). Both methods of introducing NLS-Cas9-EGFP result in a cleavage product, demonstrating the protein’s activity. Levels of the cleavage product are similar to the levels generated using our Cas9 All-in-one System, and no activity is seen in the absence of Cas9. Figure 6B shows a similar set of studies for NLS-Cas9-EGFP electroporated into target cells.

Figure 6. NLS-Cas9-EGFP protein is functional in an in vivo assay.

